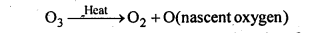Question:
Why does O3 act as a powerful oxidising agent?
Answer:
On heating, O3 readily decomposes to give O2 and nascent oxygen.
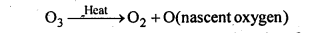
Since nascent oxygen is very reactive, therefore, O3 acts as a powerful oxidising agent.
The p-Block Elements.
Q 1.
Write the reactions of F2 and Cl2 with water.
Q 2.
Match the formulas of oxides given in Column I with the type of oxide given in Column II and mark the correct option.

Q 3.
Can PCl5 act as an oxidising as well as a reducing agent Justify.
Q 4.
Why are halogens coloured?
Q 5.
Write two uses of ClO2
Q 6.
Write balanced equations for the following:
(i) NaCl is heated witlrsulphuric acid in the presence of MnO2
(ii) Chlorine gas is passed into a solution of Nal in water.
Q 7.
Match the compounds given in Column I with the hybridization and shape given in Column II and mark the correct option.

Q 8.
Write the order of thermal stability of the – hydrides of Group 16 elements.
Q 9.
Write a balanced equation for the hydrolytic reaction of PC is in heavy water.
Q 10.
How is the presence of SO2 detected ?
Q 11.
Give the resonating structures of N02 and N2O5.
Q 12.
Write two uses of ClO2
Q 13.
What are the oxidation states of phosphorus in the following: –
(i) H3PO3 (ii)PCl3
(iii) Ca3P2(iv)Na3PO4
(v) POF3
Q 14.
Write a balanced chemical equation, for the reaction showing catalytic oxidation of NH3 by atmospheric oxygen.
Q 15.
Why is BiH3 the strongest reducing agent amongst all the hydrides of group 15 elements? (C.B.S.E. 2013)
Q 16.
What happens when sulp’hur dioxide is passed through an aqueous solution of Fe(III) salt?
Q 17.
Write the conditions to maximise the yield of H2SO4 by Contact process.
Q 18.
Why is dioxygen a gas but sulphur a solid?
Q 19.
Which one of the following does not exist ?
(i)XeOF4 (ii)NeF2
(iii)XeF4 (iv)XeF6
Q 20.
Reduction potentials of some ions are given below. Arrange them in decreasing order of oxidizing power.

Q 21.
Give reason to explain why ClF3 exists but FCl3 does not exist.
Q 22.
How is nitrogen prepared in the laboratory? Write the chemical equations of the reactions . involved.
Q 23.
Why does nitrogen show catenation properties less than phosphorus ? (C.B.S.E. Foreign 2009)
Q 24.
Why is BiH3 the strongest reducing agent amongst all the hydrides of Group 15 elements?
Q 25.
Why is N2 less reactive at room temperature?
Q 26.
List the important sources of sulphur.
Q 27.
Write the order of thermal stability of the – hydrides of Group 16 elements.
Q 28.
Which of the following does not react with oxygen directly? Zn, Ti, Pt, Fe
Q 29.
How is O3 estimated quantitatively?
Q 30.
Why does NH3 form hydrogen bond but PH3 does not?
Q 31.
Explain why NH3 is basic while BiH3 is only feebly basic.
Q 32.
Nitrogen exists as diatomic molecule and phosphorus as P4. Why?
Q 33.
What inspired N. Bartlett for carrying out reaction between Xe and PtF6?
Q 34.
With what neutral molecule is CIO– isoelectronic? Is that molecule a Lewis base?
Q 35.
List the uses of neoirand argon gases.
Q 36.
A brown ring is formed in the ring test for NO3 ion. It is due to the formation of

Q 37.
Which of the following orders are correct as per the properties mentioned against each?

Q 38.
In PCl5, phosphorus is in sp3d hybridised state but all its five bonds are not equivalent. Justify your answer with reason.
Q 39.
SF6 is known but SCl6 is not. Why?
Q 40.
How does ammonia react with a solution of Cu2+?
Q 41.
What is the covalence of nitrogen in N2O5 ?
Q 42.
Which of the following does not react with oxygen directly? Zn, Ti, Pt, Fe
Q 43.
Give two examples to show the anomalous behaviour of fluorine.
Q 44.
Give the reason for bleaching action of Cl2.
Q 45.
Name two poisonous gases which can be prepared from chlorine gas
Q 46.
Why has it been difficult to study the chemistry of radon?
Q 47.
Why is the reactivity of nitrogen different from that of phosphorus?
Q 48.
Discuss the trends in chemical reactivity of group 15 elements.
Q 49.
Illustrate how copper metal can give different products on reaction with HN03.
Q 50.
Give the resonating structures of N02 and N2O5.