

How is nitrogen prepared in the laboratory? Write the chemical equations of the reactions . involved.
In laboratory, nitrogen is prepared by heating an equimolar aqueous solution of ammonium chloride and sodium nitrite. As a result of double decomposition reaction, ammonium nitrite is formed. Ammonium nitrite is unstable and decompose to form nitrogen gas.
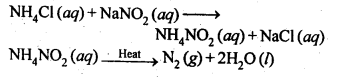
Match the formulas of oxides given in Column I with the type of oxide given in Column II and mark the correct option.

Match the compounds given in Column I with the hybridization and shape given in Column II and mark the correct option.

Assertion (A): Both rhombic and monoclinic sulphur exist as S8 but oxygen exists as OÏ€.
Reason (R): Oxygen forms pπ-pπ multiple bond due to small size and small bond length but pπ-pπ bonding is not possible in sulphur.
Write balanced equations for the following:
(i) NaCl is heated witlrsulphuric acid in the presence of MnO2
(ii) Chlorine gas is passed into a solution of Nal in water.
What happens when sulp’hur dioxide is passed through an aqueous solution of Fe(III) salt?
What are the oxidation states of phosphorus in the following: –
(i) H3PO3 (ii)PCl3
(iii) Ca3P2(iv)Na3PO4
(v) POF3
Reduction potentials of some ions are given below. Arrange them in decreasing order of oxidizing power.

Write a balanced chemical equation, for the reaction showing catalytic oxidation of NH3 by atmospheric oxygen.
In PCl5, phosphorus is in sp3d hybridised state but all its five bonds are not equivalent. Justify your answer with reason.
Why is BiH3 the strongest reducing agent amongst all the hydrides of group 15 elements? (C.B.S.E. 2013)
Why does nitrogen show catenation properties less than phosphorus ? (C.B.S.E. Foreign 2009)
Justify the placement of O, S, Se, Te and Po in the same group'of the periodic table in terms of electronic configuration, oxidation state and hydride formation.
Which of the following orders are correct as per the properties mentioned against each?

On reaction with Cl2, phosphorus forms two types of halides ‘A' and ‘B'. Halide A is yellowish-white powder but halide B' is colourless oily liquid. Identify A and B and write the formulas of their hydrolysis products.
White phosphorus reacts with chlorine and the product hydrolysis in the presence of water. Calculate the mass of HCl obtained by the hydrolysis of the product formed by the reaction of 62 g of white phosphorus with chlorine in the presence of water.
Name three oxoacids of nitrogen. Write the disproportionation reaction of that oxoacid of nitrogen in which nitrogen is in +3 oxidation state.
Nitric acid forms an oxide of nitrogen on reaction with P4O10. Write the reaction involved. Also write the resonating structures of the oxide of nitrogen formed.