

Draw the resonating structure of (i) Ozone molecule (ii) Nitrate ion

Elements X, Y and Z have 4, 5 and 7 valence electrons respectively, (i) Write the molecular formula of the compounds formed by these elements individually with hydrogen, (ii) Which of these compounds will have the highest dipole moment?
Which of the following statements are not correct?
(a) NaCl being an ionic compound is a good conductor of electricity in the solid state.
(b) In canonical structures there is a difference in the arrangement of atoms.
(c) Hybrid orbitals form stronger bonds than pure orbitals.
(d) VSEPR theory can explain the square planar geometry of XeF4.
Match the items given in Column I with examples given in Column II.
| Column I | Column II |
| (i) Hydrogen bond | (a) C |
| (ii) Resonance | (b) LiF |
| (iii) Ionic solid | (c) H2 |
| (iv) Covalent solid | (d) HF |
| (e) 03 |
Match the shape of molecules in Column I with the type of hybridization in Column II.
| Column I | Column II |
| (i) Tetrahedral | (a) sp2 |
| (ii) Trigonal | (b) sp |
| (iii) Linear | (c) sp3 |
Out of bonding and antibonding molecular orbitals, which one has lower energy and which one has higher stability?
Name the two conditions which must be satisfied for hydrogen bonding to take place in a molecule.
What is the effect of the following processes on the bond order in N-, and 02?
(i) N2 → N+2 + e– (ii) 02 → O+2 + e–
Arrange the following bonds ‘in order of increasing ionic character giving reason.
N-H, F-H, C-H and O-H
Assertion (A): Among the two O – H bonds in H20 molecule, the energy required to break the first O – H bond and other O – H bond is the same.
Reason (R): This is because the electronic environment around oxygen is the same even after breakage of one O – H bond.
(a) A and R both are correct, and R is the correct explanation of A.
(b) A and R both are correct, but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A and R both are false.
Considering X-axis as the intemuclear axis which out of the following will not form a sigma bond and why? (a) Is and Is (b) Is and 2px (c) 2py and 2py (d) Is and 2s
(a) Define dipole moment. What are the units of dipole moment?
(b) Dipole moment values help in predicting the shapes of covalent molecules. Explain.
In PO43- ion the formal charge on the oxygen atom of P – O bond is
(a) +1 (b) -1 (c) -0.75 (d) +0.75
Which of the following statements are correct about CO32- ?
(a) The hybridization of central atom is sp3.
(b) Its resonance structure has one C – O single bond and two C = O double bonds.
(c) The average formal charge on each oxygen atom is 0.67 units.
(d) All C – O bond lengths are equal.
Write Lewis structure of the following compounds and show formal charge on each atom. HN03, No2, H2so4
Explain why CO2-3 ion cannot be represented by a single Lewis structure. How can it be best represented?
Predict the shapes of the following molecules on the basis of hybridization. BC13, ch4, co2, nh3
Match the species in Column I with the type of hybrid orbitals in Column II.
| Column I | Column II |
| (i) SF4 | (a) sp3cf |
| (ii) if5 | (b) d2sp3 |
| (iii) NO2+ | (c) sp3 d |
| (iv) NH4 | (d) sp3 |
| (e) sp |
Assertion (A): Though the central atom of both NH3 and H20 molecules are sp3 hybridised, yet H – N – H bond angle is greater than that of H – O – H.
Reason (R): This is because nitrogen atom has one lone pair and oxygen atom has two lone pairs.
(a) A and R both are correct, and R is the correct explanation of A.
(b) A and R both are correct, but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A and R both are false.
Briefly describe the valence bond theory of covalent bond formation by taking an example of hydrogen. How can you interpret energy changes taking place in the formation of dihydrogen?
The skeletal structure of CH3COOH as shown below is correct, but some of the bonds are shown incorrectly. Write the correct Lewis structure for acetic acid.
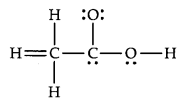
Is there any change in the hybridisation ofB and N atoms as a result of the following reaction ? BF3 + NH3 ——-> F3 B.NH3
Polarity in a molecule and hence the dipole moment depends primarily on electronegativity of the constituent atoms and shape of a molecule. Which of the following has the highest dipole moment?
(a) C02
(b) HI
(c) H20
(d) S02
Diamagnetic species are those which contain no unpaired electrons. Which among the following are diamagnetic?
(a) N2
(b) N22-
(c) 02
(d) o22-
Give reasons for the following: ‘
(a) Covalent bonds are directional bonds while ionic bonds are non- directional.
(b) Water molecule has bent structure whereas carbon dioxide molecule is linear.
(c) Ethyne molecule is linear.
Match the species in Column I with the bond order in Column II.
| Column I | , . Column II |
| (i) NO | (a) 1.5 |
| (ii) CO | (b) 2.0 |
| (iii) o–2 | (c) 2.5 |
| (iv) 02 | (d) 3.0 |
3PO3 can be represented by structures 1 and 2 shown below. Can these two structures be taken as the canonical forms of the resonance hybrid representing H3PO3? If not, give reasons for the same.
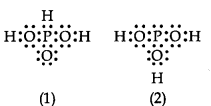
Apart from tetrahedral geometry, another possible geometry for CH4 is square planar with the four H atoms at the comers of the square and the C atom at its centre. Explain why CH4 is not square planar?