

Match the species in Column I with the geometry/shape in Column II.
| Column I | Column II |
| (i) H30+ | (a) Linear |
| (ii) HC = CH | (b) Angular |
| (iii) Cl0–2 | (c) Tetrahedral |
| (iv) NH+4 | (d) Trigonal bipyramidal |
| – | (e) Pyramidal |

Assertion (A): Though the central atom of both NH3 and H20 molecules are sp3 hybridised, yet H – N – H bond angle is greater than that of H – O – H.
Reason (R): This is because nitrogen atom has one lone pair and oxygen atom has two lone pairs.
(a) A and R both are correct, and R is the correct explanation of A.
(b) A and R both are correct, but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A and R both are false.
Elements X, Y and Z have 4, 5 and 7 valence electrons respectively, (i) Write the molecular formula of the compounds formed by these elements individually with hydrogen, (ii) Which of these compounds will have the highest dipole moment?
Match the items given in Column I with examples given in Column II.
| Column I | Column II |
| (i) Hydrogen bond | (a) C |
| (ii) Resonance | (b) LiF |
| (iii) Ionic solid | (c) H2 |
| (iv) Covalent solid | (d) HF |
| (e) 03 |
(a) How many a and n bonds are present in
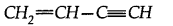
(b) Why Hf is more stable than H2?
(c) Why is B2 molecule paramagnetic?
Although both CO2 and H2O are triatomic molecules, the shape of H2O molecule is bent while that of CO2 is linear. Explain this on the basis of dipole moment.
Which of the following statements are not correct?
(a) NaCl being an ionic compound is a good conductor of electricity in the solid state.
(b) In canonical structures there is a difference in the arrangement of atoms.
(c) Hybrid orbitals form stronger bonds than pure orbitals.
(d) VSEPR theory can explain the square planar geometry of XeF4.
Why does type of overlap given in the following figure not result in the bond formation?

The skeletal structure of CH3COOH as shown below is correct, but some of the bonds are shown incorrectly. Write the correct Lewis structure for acetic acid.
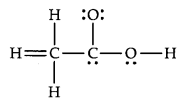
Assertion (A): Among the two O – H bonds in H20 molecule, the energy required to break the first O – H bond and other O – H bond is the same.
Reason (R): This is because the electronic environment around oxygen is the same even after breakage of one O – H bond.
(a) A and R both are correct, and R is the correct explanation of A.
(b) A and R both are correct, but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A and R both are false.
Draw the Lewis structures for the following molecules and ions:
H2S, SiCl4 , BeF2, C032-, HCOOH
Compare the relative stability of the following species and indicate their magnetic properties: O2, O2, O2– (Superoxide),O22- (peroxide)
Account for the following:
(i) Water is a liquid while H2S is a gas
(ii) NH3 has higher boiling point than PH3.
Using molecular orbital theory, compare the bond energy and magnetic character of 0+2 and O–2
Arrange the following bonds ‘in order of increasing ionic character giving reason.
N-H, F-H, C-H and O-H
Is there any change in the hybridisation ofB and N atoms as a result of the following reaction ? BF3 + NH3 ——-> F3 B.NH3
What is the total number of sigma and pi bonds in the following molecules?
(a) C2 H2 (b) C2 H4
Considering X-axis as the intemuclear axis which out of the following will not form a sigma bond and why? (a) Is and Is (b) Is and 2px (c) 2py and 2py (d) Is and 2s
Name the two conditions which must be satisfied for hydrogen bonding to take place in a molecule.
Write Lewis structure of the following compounds and show formal charge on each atom. HN03, No2, H2so4
What is an ionic bond? With two suitable examples explain the difference between an ionic and covalent bond?
Explain why CO2-3 ion cannot be represented by a single Lewis structure. How can it be best represented?
Explain why BeH2 molecule has a zero dipole moment although the Be—H bonds are polar.
Write the important conditions required for the linear combination of atomic orbitals to form molecular orbitals.