

Briefly describe the valence bond theory of covalent bond formation by taking an example of hydrogen. How can you interpret energy changes taking place in the formation of dihydrogen?
The valence bond theory was put forward by Heitler and London in 1927. It was later improved and developed by L. Pauling and J.C. Slater in 1931. The valence bond theory is based on the knowledge of atomic orbitals and electronic configurations of elements, overlap criteria of atomic orbitals and stability of molecule.
The main points of valence bond theory are
(i) Atoms do not lose their identity even after the formation of the molecule.
(ii) The bond is formed due to the interaction of only the valence electrons as the two atoms come close to each other. The inner electrons do not participate in the bond formation.
(iii) During the formation of bond, only the valence electrons from each bonded atom lose their identity. The other electrons remain unaffected.
(iv) The stability of bond is accounted by the fact that the formation of bond is accompanied by release of energy. The molecule has minimum energy at a certain distance between the atoms known as intemuclear distance. Larger the decrease in energy, stronger will be the bond formed.
Valence bond Treatment of Hydrogen Molecule:
Consider two hydrogen atoms A and B approaching each other havingnuclei Ha and HB and the corresponding electrons eA and eB respectively.
When atoms come closer to form molecules new forces begin to operate.
(a) The force of attraction between nucleus of atom and electron of another atom.
(b) The force of repulsion between two nuclei of the atom and electron of two atoms.


Fig. (a) Two hydrogen atoms at a large distance and hence, no interaction, (b) Two hydrogen atom closer to each other atomic orbitals begin to interact, (c) Attractive and repulsive forces in hydrogen atoms when interaction begins. In case of hydrogen: Figure ‘a' shows that two hydrogen atoms are at farthest distances and their electron distribution is absolutely symmetrical.
(a) When two hydrogen atom start coming closer to each other, the electron cloud becomes distorted and new attractive and repulsive forces begin to operate as shown in figure ‘c'
(b) In figure ‘c' dotted lines show attractive forces present in atom already and bold lines show the new attractive and repulsive forces.
(c) It has been found experimentally that the magnitude of net attractive forces is more than net repulsive forces. Thus stable hydrogen molecule is formed.
Potential energy diagram for formation of hydrogen molecules:
When two hydrogen atoms are at farther distance, there is no force operating between them, when they start coming closer to each other, force of attraction comes into play and their potential energy starts decreasing. As they come closer to each other potential goes on decreasing, but a point is reached, when potential energy acquires minimum value.
Note:
(a) This distance corresponding to this minimum energy value is called the distance of maximum possible approach, i.e. the point which corresponds to minimum energy and maximum stability.
(b) If atoms come further closer than this distance of maximum possible approach, then potential energy starts increasing and force of repulsion comes into play and molecules starts becoming unstable.

Elements X, Y and Z have 4, 5 and 7 valence electrons respectively, (i) Write the molecular formula of the compounds formed by these elements individually with hydrogen, (ii) Which of these compounds will have the highest dipole moment?
Which of the following statements are not correct?
(a) NaCl being an ionic compound is a good conductor of electricity in the solid state.
(b) In canonical structures there is a difference in the arrangement of atoms.
(c) Hybrid orbitals form stronger bonds than pure orbitals.
(d) VSEPR theory can explain the square planar geometry of XeF4.
Match the items given in Column I with examples given in Column II.
| Column I | Column II |
| (i) Hydrogen bond | (a) C |
| (ii) Resonance | (b) LiF |
| (iii) Ionic solid | (c) H2 |
| (iv) Covalent solid | (d) HF |
| (e) 03 |
Is there any change in the hybridisation ofB and N atoms as a result of the following reaction ? BF3 + NH3 ——-> F3 B.NH3
Considering X-axis as the intemuclear axis which out of the following will not form a sigma bond and why? (a) Is and Is (b) Is and 2px (c) 2py and 2py (d) Is and 2s
Which of the following statements are correct about CO32- ?
(a) The hybridization of central atom is sp3.
(b) Its resonance structure has one C – O single bond and two C = O double bonds.
(c) The average formal charge on each oxygen atom is 0.67 units.
(d) All C – O bond lengths are equal.
Write Lewis structure of the following compounds and show formal charge on each atom. HN03, No2, H2so4
What is an ionic bond? With two suitable examples explain the difference between an ionic and covalent bond?
Assertion (A): Though the central atom of both NH3 and H20 molecules are sp3 hybridised, yet H – N – H bond angle is greater than that of H – O – H.
Reason (R): This is because nitrogen atom has one lone pair and oxygen atom has two lone pairs.
(a) A and R both are correct, and R is the correct explanation of A.
(b) A and R both are correct, but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A and R both are false.
Briefly describe the valence bond theory of covalent bond formation by taking an example of hydrogen. How can you interpret energy changes taking place in the formation of dihydrogen?
Write Lewis symbols for the following atoms and ions: S and S2– ; Al and Al3+; H and H–
Out of bonding and antibonding molecular orbitals, which one has lower energy and which one has higher stability?
Name the two conditions which must be satisfied for hydrogen bonding to take place in a molecule.
(a) Define dipole moment. What are the units of dipole moment?
(b) Dipole moment values help in predicting the shapes of covalent molecules. Explain.
(a) How many a and n bonds are present in
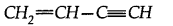
(b) Why Hf is more stable than H2?
(c) Why is B2 molecule paramagnetic?
Arrange the following bonds ‘in order of increasing ionic character giving reason.
N-H, F-H, C-H and O-H
Arrange the bonds in order of increasing ionic character in the molecules: LiF, K2O, N2, SO2 and ClF3.
The skeletal structure of CH3COOH as shown below is correct, but some of the bonds are shown incorrectly. Write the correct Lewis structure for acetic acid.
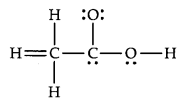
Apart from tetrahedral geometry, another possible geometry for CH4 is square planar with the four H atoms at the comers of the square and the C atom at its centre. Explain why CH4 is not square planar?
What is the total number of sigma and pi bonds in the following molecules?
(a) C2 H2 (b) C2 H4
Define Lattice energy. How is Lattice energy influenced by (i) Charge on the ions (ii) Size of the ions?
Account for the following:
(i) Water is a liquid while H2S is a gas
(ii) NH3 has higher boiling point than PH3.
Explain the diamagnetic behaviour of P2 molecule on the basis of molecular orbital theory.
Apart from tetrahedral geometry, another possible geometry for CH4 is square planar with the four H atoms at the comers of the square and the C atoms at its centre. Explain why CH4 is not square planar?
Which molecule/ion out of the following does not contain unpaired electrons?
(a) N+2
(b) 02
(c) O22-
(d) B2
Diamagnetic species are those which contain no unpaired electrons. Which among the following are diamagnetic?
(a) N2
(b) N22-
(c) 02
(d) o22-
Explain the non linear shape of H2S and non planar shape of PCl3 using valence shell electron pair repulsion theory.