

Why does NH3 form hydrogen bond but PH3 does not?
Nitrogen has an electronegativity value 3.0, which is much higher than that of H (2.1). As a result, N – H bond is quite polar and hence NH3 undergoes intermolecular H – bonding.
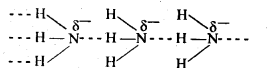
Phosphorus have an electronegativity value 2-1. Thus, P – H bond is not polar and hence PH3 does not undergo H – bonding.
Match the formulas of oxides given in Column I with the type of oxide given in Column II and mark the correct option.

Match the compounds given in Column I with the hybridization and shape given in Column II and mark the correct option.

Write balanced equations for the following:
(i) NaCl is heated witlrsulphuric acid in the presence of MnO2
(ii) Chlorine gas is passed into a solution of Nal in water.
What are the oxidation states of phosphorus in the following: –
(i) H3PO3 (ii)PCl3
(iii) Ca3P2(iv)Na3PO4
(v) POF3
Write a balanced chemical equation, for the reaction showing catalytic oxidation of NH3 by atmospheric oxygen.
Assertion (A): Both rhombic and monoclinic sulphur exist as S8 but oxygen exists as OÏ€.
Reason (R): Oxygen forms pπ-pπ multiple bond due to small size and small bond length but pπ-pπ bonding is not possible in sulphur.
Why is BiH3 the strongest reducing agent amongst all the hydrides of group 15 elements? (C.B.S.E. 2013)
What happens when sulp’hur dioxide is passed through an aqueous solution of Fe(III) salt?
Why does nitrogen show catenation properties less than phosphorus ? (C.B.S.E. Foreign 2009)
Reduction potentials of some ions are given below. Arrange them in decreasing order of oxidizing power.

Which of the following orders are correct as per the properties mentioned against each?

How is nitrogen prepared in the laboratory? Write the chemical equations of the reactions . involved.
The HNH angle value is higher than HPH, H AsH and HSbH angles. Why?
(Hint: Can be explained on the basis of sp3 hybridisation in NH3 and only s-p bonding , between hydrogen and other elements of the group).
Explain why inspite of nearly the same electronegativity, nitrogen forms hydrogen bonding while chlorine does not.
Why is BiH3 the strongest reducing agent amongst all the hydrides of Group 15 elements?
What happens when white phosphorus is heated with concentrated NaOH solution in an inert atmosphere of CO2 ?
What happens when sulp'hur dioxide is passed through an aqueous solution of Fe(III) salt?
Comment on the nature of two S-O bonds formed in S02 molecule. Are the two S-O bonds in this molecule equal ?