

Why does NH3 form hydrogen bond but PH3 does not?
Nitrogen has an electronegativity value 3.0, which is much higher than that of H (2.1). As a result, N – H bond is quite polar and hence NH3 undergoes intermolecular H – bonding.
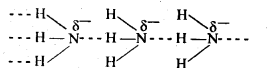
Phosphorus have an electronegativity value 2-1. Thus, P – H bond is not polar and hence PH3 does not undergo H – bonding.
With which neutral molecule is ClO– isoelectronic? Is this molecule Lewis acid or base ? (Pb. Board 2009)
Which of the following acid forms three series of salts?
(a) H2PO2 (b) H3BO3 (C)H3PO4(d)H3PO3
Nitric acid forms an oxide of nitrogen on reaction with P4O10. Write the reaction involved. Also write the resonating structures of the oxide of nitrogen formed.
Why is BiH3 the strongest reducing agent amongst all the hydrides of group 15 elements? (C.B.S.E. 2013)
Why does nitrogen show catenation properties less than phosphorus ? (C.B.S.E. Foreign 2009)
Write a balanced chemical equation, for the reaction showing catalytic oxidation of NH3 by atmospheric oxygen.
Phosphorus forms a number of oxoacids. Out of these oxoacids phosphinic acid has strong reducing property. Write its structure and also write a reaction showing its reducing behaviour.
Assertion (A): HI cannot be prepared by the reaction of KI with concentrated H2SO4.
Reason (R): HI has lowest H – X bond strength among halogen acids.
Assertion (A): NaCl reacts with concentrated H2SO4 to give colourless fumes with pungent smell. But on adding MnO2 the fumes become greenish yellow.
Reason (R): MnO2 oxidises HCl to chlorine gas which is greenish yellow.
What happens when sulp’hur dioxide is passed through an aqueous solution of Fe(III) salt?
Write balanced equations for the following:
(i) NaCl is heated witlrsulphuric acid in the presence of MnO2
(ii) Chlorine gas is passed into a solution of Nal in water.
How is nitrogen prepared in the laboratory? Write the chemical equations of the reactions . involved.
On heating ammonium dichromate and barium azide separately we get
(a) N2 in both cases
(b) N2 with ammonium dichromate and NO with barium azide
(c) N2O with ammonium dichromate and N2 with barium azide
(d) N2O with ammonium dichromate and N2O with barium azide
Which of the following statements are correct?
(a) Among halogens, radius ratio between iodine and fluorine is maximum.
(b) Leaving F – F bond, all halogens have weaker X – X bond than X – X’ bond in interhalogens.
(c) Among interhalogen compounds maximum number of atoms ate present in iodine fluoride.
(d) Interhalogen compounds are more reactive than halogen compounds.
Which of the following statements are correct?
(a) All three N – O bond lengths in HNO3 are equal.
(b) All P – Cl bond lengths in PCl5 molecule in gaseous state are equal.
(c) P4 molecule in white phosphorus have angular strain therefore white phosphorus is very reactive.
(d) PCl5 is ionic in solid state in which cation is tetrahedral and anion is octahedral.
Which of the following statements are true?
(a) Only type of interactions between particles of noble gases are due to weak dispersion forces.
(b) Ionisation enthalpy of.molecular oxygen is very close to that of xenon.
(c) Hydrolysis of XeF6 is a redox reaction.
(d) Xenon fluorides are not reactive.
Explain why does the stability of oxoacids of chlorine increase in the order given below:
HClO < HClO2 < HClO3 < HClO4
Phosphorus has three allotropic fonns —(i) white phosphorus (ii) red phosphorus and (iii) black phosphorus. Write the difference between white and red phosphorus on the basis of their structure and reactivity.
Match the formulas of oxides given in Column I with the type of oxide given in Column II and mark the correct option.

Assertion (A): HNO3 makes from passive.
Reason (R): HNO3 forms a protective layer of ferric nitrate on the surface of iron.
Comment on the nature of two S-O bonds formed in S02 molecule. Are the two S-O bonds in this molecule equal ?
Explain why inspite of nearly the same electronegativity, nitrogen forms hydrogen bonding while chlorine does not.