

Why is bond angle in PH+4 ion higher than in PH3 ? (Pb. Board 2009)
In both PH3 and PH+4 ion, the phosphorus atom is sp3 hybridised. However, in PH3 the central atom has apyramidal structure due to the presence of lone electron pair on the phosphorus atom.
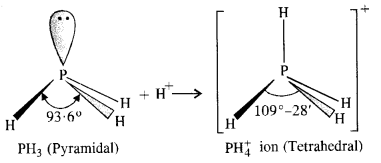
Because of lone pair : shared pair repulsion which is more than that of shared pair : shared pair repulsion, the bond angle in PH3 is nearly 93-6°. In PH+4 ion, there is no lone electron pair on the phosphorus atom. It has a tetrahedral structure with bond angle of 109°-28′. Thus, the bond angle in PH+4 ion is higher than in PH3.
Write balanced equations for the following:
(i) NaCl is heated witlrsulphuric acid in the presence of MnO2
(ii) Chlorine gas is passed into a solution of Nal in water.
Which of the following statements are true?
(a) Only type of interactions between particles of noble gases are due to weak dispersion forces.
(b) Ionisation enthalpy of.molecular oxygen is very close to that of xenon.
(c) Hydrolysis of XeF6 is a redox reaction.
(d) Xenon fluorides are not reactive.
In PCl5, phosphorus is in sp3d hybridised state but all its five bonds are not equivalent. Justify your answer with reason.
In the ring test of NO3 ion.Fe2+ion reduces nitrate ion to nitric oxide, which combines with Fe2+ (aq.) ion to form brown complex. Write the reactions involved in the formation of brown ring.
Why does nitrogen show catenation properties less than phosphorus ? (C.B.S.E. Foreign 2009)
Explain why does the stability of oxoacids of chlorine increase in the order given below:
HClO < HClO2 < HClO3 < HClO4
Why is BiH3 the strongest reducing agent amongst all the hydrides of group 15 elements? (C.B.S.E. 2013)
What happens when sulp’hur dioxide is passed through an aqueous solution of Fe(III) salt?
Knowing the electron gain enthalpy values of O—>O– and O—>O2- as -141 and 702 kJ mol-1 respectively, how can you account for the formation of a large number of oxides having O2- species and not O–?
With which neutral molecule is ClO– isoelectronic? Is this molecule Lewis acid or base ? (Pb. Board 2009)
What happens when white phosphorus is heated with concentrated NaOH solution in an inert atmosphere of CO2 ?
How is nitrogen prepared in the laboratory? Write the chemical equations of the reactions . involved.
Match the compounds given in Column I with the hybridization and shape given in Column II and mark the correct option.

On heating compound (A) gives a gas (B) which is a constituent of air. This gas when treated with 3 mol of hydrogen (H2 ) in the presence of a catalyst gives another gas (C) which is basic in nature. Gas C on further oxidation in moist condition gives a compound (D) which is a part of acid rain. Identify compounds (A) to (D) and also give necessary equations of all the steps involved. –
Explain why inspite of nearly the same electronegativity, nitrogen forms hydrogen bonding while chlorine does not.
Why is BiH3 the strongest reducing agent amongst all the hydrides of Group 15 elements?