

Describe the hybridisation in case of PCl5. Why are the axial bonds longer as compared to equatorial bonds?
The ground state E.C. and the excited state E.C. of phosphorus are represented as:
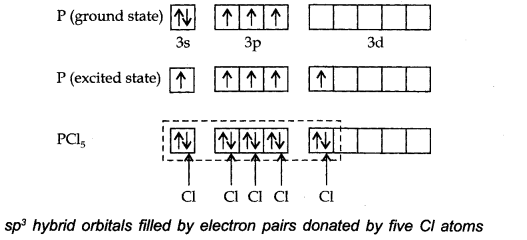
The one s, three-p and one d-orbitals hybridise to yield five sets of SP3 d hybrid orbitals which are directed towards the five corners of a trigonal bipyramidal as in Fig.
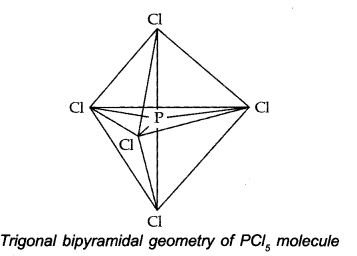
Because axial bond pairs suffer more repulsive interaction from the equatorial bond pairs, therefore axial bonds have been found to be slightly longer and hence slightly weaker than equatorial bonds.
Match the species in Column I with the geometry/shape in Column II.
| Column I | Column II |
| (i) H30+ | (a) Linear |
| (ii) HC = CH | (b) Angular |
| (iii) Cl0–2 | (c) Tetrahedral |
| (iv) NH+4 | (d) Trigonal bipyramidal |
| – | (e) Pyramidal |
In which of the following substances will hydrogen bond be strongest?
(a) HCl
(b) H20
(c) HI
(d) H2S
Which of the following statements are correct about CO32- ?
(a) The hybridization of central atom is sp3.
(b) Its resonance structure has one C – O single bond and two C = O double bonds.
(c) The average formal charge on each oxygen atom is 0.67 units.
(d) All C – O bond lengths are equal.
Match the shape of molecules in Column I with the type of hybridization in Column II.
| Column I | Column II |
| (i) Tetrahedral | (a) sp2 |
| (ii) Trigonal | (b) sp |
| (iii) Linear | (c) sp3 |
Which hybrid orbitals are used by carbon atoms in the following molecules?
(a) CH3-CH3 (b) CH3-CH = CH2 (c) CH3-CH2-OH (d) CH3-CHO (e) CH3COOH.
Account for the following:
(i) Water is a liquid while H2S is a gas
(ii) NH3 has higher boiling point than PH3.
Using molecular orbital theory, compare the bond energy and magnetic character of 0+2 and O–2
The skeletal structure of CH3COOH as shown below is correct, but some of the bonds are shown incorrectly. Write the correct Lewis structure for acetic acid.
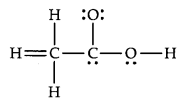
Name the two conditions which must be satisfied for hydrogen bonding to take place in a molecule.
3PO3 can be represented by structures 1 and 2 shown below. Can these two structures be taken as the canonical forms of the resonance hybrid representing H3PO3? If not, give reasons for the same.
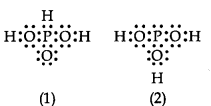
(a) How many a and n bonds are present in
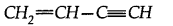
(b) Why Hf is more stable than H2?
(c) Why is B2 molecule paramagnetic?
In N0–3 ion, the number of bond pairs and lone pairs of electrons on nitrogen atom are
(a) 2, 2 (b) 3, 1 (c) 1,3 (d) 4, 0
What is meant by hybridisation of atomic orbitals? Describe the shapes of sp, sp2, sp3 hybrid orbitals.
Explain the diamagnetic behaviour of P2 molecule on the basis of molecular orbital theory.
Predict the shapes of the following molecules on the basis of hybridization. BC13, ch4, co2, nh3
Draw diagrams showing the formation of a double bond and a triple bond between carbon atoms in C2 H4 and C2 H2 molecules.
Explain the non linear shape of H2S and non planar shape of PCl3 using valence shell electron pair repulsion theory.
Use Lewis symbols to show electron transfer between the following atoms to form cations and anions (a) K and S (b) Ca and O (c) Al and N.
Define Lattice energy. How is Lattice energy influenced by (i) Charge on the ions (ii) Size of the ions?
Apart from tetrahedral geometry, another possible geometry for CH4 is square planar with the four H atoms at the comers of the square and the C atoms at its centre. Explain why CH4 is not square planar?
Why does type of overlap given in the following figure not result in the bond formation?

What is an ionic bond? With two suitable examples explain the difference between an ionic and covalent bond?
Explain why CO2-3 ion cannot be represented by a single Lewis structure. How can it be best represented?
What is meant by the term average bond enthalpy? Why there is difference in bond enthalpy of O – H bond in ethanol (C2H5OH) and water?
Match the species in Column I with the type of hybrid orbitals in Column II.
| Column I | Column II |
| (i) SF4 | (a) sp3cf |
| (ii) if5 | (b) d2sp3 |
| (iii) NO2+ | (c) sp3 d |
| (iv) NH4 | (d) sp3 |
| (e) sp |