

An O2 cylinder has 10 LO2 at 200 atm. If patient takes 0.50 ml of O2 at 1 atm in one breath 37 °C, how many breaths are possible?
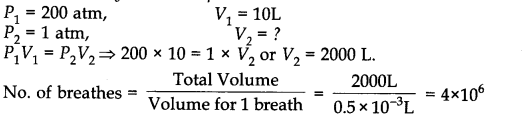
Which of the following changes decrease the vapour pressure of water kept in a sealed vessel?
(a) Decreasing the quantity of water
(b) Adding salt to water
(c) Decreasing the volume of the vessel to one-half
(d) Decreasing the temperature of water
What will be the pressure of the gas mixture when 0.5 L of H2 at 0.8 bar and 2.0 L of dioxygen at 0.7 bar are introduced in all vessel at 27 °C?
With regard to the gaseous state of matter which of the following statements are correct?
(a) Complete order of molecules (b) Complete disorder of molecules
(c) Random motion of molecules (d) Fixed position of molecules
One of the assumptions of kinetic theory of gases states that "there is no force of attraction between the molecules of a gas."How far is this statement correct? Is it possible to liquefy an ideal gas? Explain.
The critical temperature (Tc) and critical pressure (Pc) of C02 are 30.98 °C and 73 atm respectively. Can C02(g) be liquefied at 32 °C and 80 atm pressure?
2.9 g of a gas at 95 °C occupied the same volume as 0.184 g of hydrogen at 17 °C at the same pressure. What is the molar mass of the gas ?
Which of the following figures does not represent 1 mole of dioxygen gas at STP?
(a) 16 grams of gas
(b) 22.7 litres of gas
(c) 6.022 x 1023 dioxygen molecules
(d) 11.2 litres of gas
Assertion (A): Three states of matter are the result of balance between intermolecular forces and thermal energy of the molecules. .
Reason (R): Intermolecular forces tend to keep the molecules together but thermal energy of molecules tends to keep them apart.
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false but R is true.
Assertion (A): The temperature at which vapour pressure of a liquid is equal to the external pressure is called boiling temperature.
Reason (R): At high altitude atmospheric pressure is high.
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false but R is true.
Isotherms of carbon dioxide at various temperatures are represented in the following figure. Answer the following questions based on this figure.

(i) In which state will C02 exist between the points a and b at temperature T1
(ii) At what point will Co2 start liquefying when temperature is T1?
(iii) At what point will C02 be completely liquefied when temperature is T2?
(iv) Will condensation take place when the temperature is T3
(v) What portion of the isotherm at T1 represent liquid and gaseous C02 at equilibrium?
Why does the boundary between liquid phase and gaseous phase disappear on heating a liquid up to critical temperature in a closed vessel? In this situation what will be the state of the substance?
(a) What do you mean by’Surface Tension'of a liquid?
(b) Explain the factors which can affect the surface tension of a liquid.
Two different gases ˜A' and ˜9' are filled in separate containers of equal capacity under the same conditions of temperature and pressure. On increasing the pressure slightly, the gas ˜A' liquefies but gas ˜B' does not liquefy even on applying high pressure until it is cooled. Explain this phenomenon.
State and explain Dalton’s law of partial pressures. Can we apply Dalton's law of partial pressures to a mixture of carbon monoxide and oxygen?
Use the information and data given below to answer the questions (a) to (c):
• Stronger intermolecular forces result in higher boiling point.
• Strength of London forces increases with the number of electrons in the molecule.
• Boiling point of HF, HC1, HBr and HI are 293 K, 189 K, 206 K and 238 K respectively.
(a) Which type of intermolecular forces are present in the molecules HF, HC1, HBr and HI?
(b) Looking at the trend of boiling points of HC1, HBr and HI, explain out of dipole-dipole interaction and London interaction, which one is predominant here?
(c) Why is boiling point of hydrogen fluoride highest while that of hydrogen chloride lowest?
The variation of vapour pressure of different liquids with temperature is shown in figure

(i) Calculate graphically boiling points of liquids A and B.
(ii) If we take liquid C in a closed vessel and heat it continuously, at what temperature will it boil?
(iii) At high altitude, atmospheric pressure is low (say 60 mm Hg). At what temperature liquid D boils?
(iv) Pressure cooker is used for cooking food at hill station. Explain in terms of vapour pressure why is it so?
Critical temperature for Co2 and CH4 are 31.1 °C and -81.9 °C respectively. Which of these has stronger intermolecular forces and why ?
How is compressibility factor expressed in terms of molar volume of the real gas and that of the ideal gas?
(a) Why aerated water bottles kept under water during summer?
(b) Which property of liquid is responsible for spherical shape of drop?
(c) Why is moist air lighter than dry air?
(d) Define aqueous tension.
(e) What are units of a and b which are van der Waals constants?
One of the assumptions of kinetic theory of gases is that there is no force of attraction between the molecules of a gas.
State and explain the evidence that shows that the assumption is not applicable for real gases.
Match the graph between the following variables with their names.
| Column I (Graphs) | Column II (Names) |
| (i) Pressure vs temperature graph at constant molar volume. | (a) Isotherms |
| (ii) Pressure vs volume graph at constant temperature. | (b) Constant temperature curve |
| (iii) Volume vs temperature graph at constant pressure. | (c) Isochores |
| (d) Isobars |
Calculate the temperature of 4.0 moles of a gas occupying 5 dm3 at 3.32 bar (R = 0.083 bar dm3 K-1 mol-1)
Name the energy which arises due to motion of atoms or molecules in a body. How is this energy affected when the temperature is increased?
For real gases the relation between P, V and T is given by van der Waals equation:

where ‘a' and ‘b' are van der Waals constants, ‘nb' is approximately equal to the total volume of the molecules of a gas.
‘a' is the measure of magnitude of intermolecular attraction.
(i) Arrange the following gases in the increasing order of ‘b'. Give reason. 02, C02, H2, He
(ii) Arrange the following gases in the decreasing order of magnitude of ‘a'. Give reason.CH4, O2, H2
Assertion (A): At constant temperature, PV vs V plot for real gases is not a straight line.
Reason (R): At high pressure all gases have Z> 1 but at intermediate pressure most gases have Z < 1.
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false but R is true.
Assertion (A): Gases do not liquefy above their critical temperature, even on applying high pressure.
Reason (R): Above critical temperature, the molecular speed is high and intermolecular attractions cannot hold the molecules together because they escape because of high speed.
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false but R is true.
Assertion (A): Liquids tend to have maximum number of molecules at their surface.
Reason (R): Small liquid drops have spherical shape.
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false but R is true.
A weather balloon has a volume of 175 dm3 when filled with hydrogen gas at a pressure of 1.0 bar. Calculate the volume of the balloon when it rises to a height where the atmospheric pressure is 0.8 bar. Assume that temperature is constant.
At 25 °C and 760 mm ofHg pressure a gas occupies 600 mL volume. What will be its pressure at a height where temperature is 10 °C and volume of the gas is 640 mL?
Under which of the following two conditions applied together, a gas deviates most from the ideal behaviour?
(a) Low pressure (b) High pressure
(c) Low temperature (d) High temperature
The behaviour of matter in different states is governed by various physical laws. According to you what are the factors that determine the state of matter?
A gas that follows Boyle's law, Charles' law and Avogadro's law is called an ideal gas. Under what conditions a real gas would behave ideally?