

Write balanced chemical equations for the following reactions:
(i) Permanganate ion (Mn0–4) reacts with sulphur dioxide gas in acidic medium to produce Mn2+ and hydrogensulphate ion. (Balance by ion- electron method)
(ii) Reaction of liquid hydrazine (N2H4) with chlorate ion (C10–3) in basic medium produces nitric oxide gas and chloride ion in gaseous state. (Balance by oxidation number method)
(iii) Dichlorine heptaoxide (C1207) in gaseous state combines with an aqueous solution of hydrogen peroxide in acidic medium to give chlorite ion (C10–2) and oxygen gas. (Balance by ion-electron method)
(i) 2Mn0–4 + 5S02 + 2H20 + H+→5HS0–4 + 2Mn2+
Balancing by ion-electron method:




Identify the correct statements with reference to the given reaction.
P4 + 30H– + 3H20→ PH3 + 3H2 P0–2
(a) Phosphorus is undergoing reduction only.
(b) Phosphorus is undergoing oxidation only.
(c) Phosphorus is undergoing oxidation as well as reduction.
(d) Hydrogen is undergoing neither oxidation nor reduction
While sulphur dioxide and hydrogen peroxide can act as an oxidising as well as reducing agents in their reactions, ozone and nitric acid act only as oxidants. Why?
Calculate the oxidation number of phosphorus in the following species.
(a) HPO32- and (b) P043-
In Ostwald’s process for the manufacture of nitric add, the first step involves the oxidation of ammonia gas by oxygen gas to give nitric oxide gas and steam. What is the maximum wight of nitric oxide that can be obtained starting only with 10.0 g of ammonia and 20.0 g of oxygen?
Arrange the following metals in the order in which they displace each other from the solution of their salts.Al, Cu, Fe, Mg and Zn.
Predict the products of electrolysis in each of the folloxving:
(i) An aqueous solution of AgNO3 with silver electrodes.
(ii) An aqueous solution of silver nitrate with platinum electrodes.
(iii) A dilute solution of H2S04with platinum electrodes.
(iv) An aqueous solution of CuCl2 with platinum electrodes.
Which of the following statement(s) is/are not true about the following decomposition reaction?
2KCIO3 →2KC1 + 302
(a) Potassium is undergoing oxidation.
(b) Chlorine is undergoing oxidation.
(c) Oxygen is reduced.
(d) None of the species are undergoing oxidation or reduction.
Which of the following electrodes will act as anodes, when connected to Standard Hydrogen Electrode?
(a) A13-/A1; E °= -1.66 V
(b) Fe2+ /Fe; E °= -0.44 V
(c) Cu2+/ Cu E °=34 V
(d) F2(g)/2F–(aq) E °= 2.87 V
Justify-giving reactions that among halogens, fluorine is the best oxidant and among hydrohalic compounds, hydroiodic add is the best reductant.
(a) Balance the following equation by oxidation number method or by ion electron (half reaction) method.
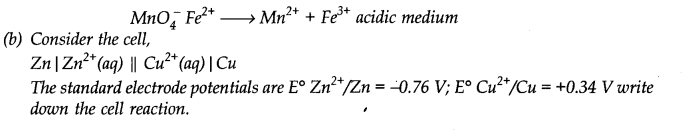
Calculate the oxidation number of each sulphur atom in the following compounds:
(a) Na2S203
(b) Na2S406
(c) Na2S03
(d) Na2S04
Fluorine reacts with ice and results in the change:
H20(S) + F2 (g) ——-> HF(g) + HOF(g)
Justify that this reaction is a redox reaction.
The reaction Cl2(g) + 20H–(aq)→ Cl0–(aq) + Cl–(aq) + H20(l) represents the process of bleaching. Identify and name the species that bleaches the substances due to its oxidizing action.
The compound AgF2 is unstable. However, if formed, the compound acts as a very strong oxidising agent. Why?
Depict the galvanic cell in which the reaction, Zn(s) + 2Ag+(aq) ————> Zn2+(aq) + 2Ag(s)
takes place. Further show:
(i) which of the electrode is negatively charged.
(ii) the carriers of current in the cell and
(iii) individual reaction at each electrode.
Which of the following elements does not show disproportionation tendency?
(a) Cl
(b) Br
(c) F
(d) I
Identify the redox reactions out of the following reactions and identify the oxidizing and reducing agents in them

Identify the substance oxidised, reduced, oxidising agent and reducing agent for each of the following reactions.
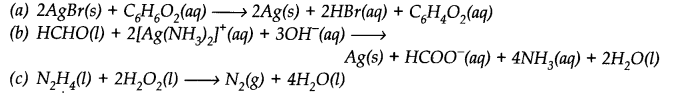
Identify the correct statement(s) in relation to the following reaction:
Zn + 2HCl → ZnCl2 + H2
(a) Zinc is acting as an oxidant.
(b) Chlorine is acting as a reductant.
(c) Hydrogen ion is acting as an oxidant.
(d) Zinc is acting as a reductant.

The exhibition of various oxidation states by an element is also related to the outer orbital electronic configuration of its atom. Atom(s) having which of the following outermost electronic configurations will exhibit more than one oxidation state in its/their compounds.
(a) 3s1
(b) 3dl4s2
(c) 3d24s2
(d) 3s23p3
Write Jour informations about the reaction:
(CN)2(g) + 2OH–(aq) —–> CN–(aq) + CNO–(aq) + H2O(l)
Identify the substance oxidised, reduced, oxidising agent and reducing agent for each of the following reactions.
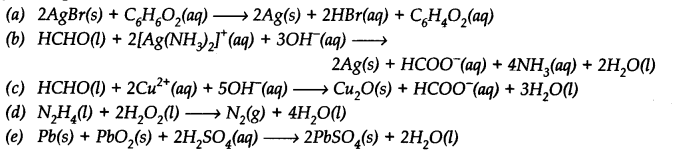
What is standard hydrogen electrode? For what purpose it is used? What are signs of oxidation potential and reduction potential decided by using SHE (Standard hydrogen electrode)?
In which of the following compounds, an element exhibits two different oxidation states.
(a) NH2OH
(b) NH4NO3
(c) N2H4
(d) N3H
Write formulas for the following compounds:
(a) Mercury (II) chloride, (b) Nickel (II) sulphate, (c) Tin (IV) oxide, (d) Thallium
(I) sulphate, (e) Iron (III) sulphate, (f) Chromium (III) oxide.
Balance the following redox reactions by ion-electron method.
(a) MnO4–(aq) +I–(aq) ———>Mn02(s) + I2 (s) (in basic medium)
(b) MnO4–(aq) + S02(g) ——-> Mn2+(aq) +H2S04–(in acidic solution)
(c) H2O2(aq) + Fe2+(aq) ———-> Fe3+(aq) + H2O(l) (in acidic solution)
(d) Cr2O72- (aq) + S02 (g)——> Cr3+ (aq) + SO42-(aq) (in acidic solution)
PbO and Pb02 react with HC1 according to following chemical equations:
2PbO + 4HCl → 2PbCl2 + 2H20
Pb02 + 4HC1 → PbCl2 + Cl2 + 2H20
Why do these compounds differ in their reactivity?
What are the oxidation number of the underlined elements in each of the following and how do you rationalise your results ?

Justify that the following reactions are redox reactions:
(a) CuO(s) + H2(g) —–> Cu(s) + H20(g)
(b) Fe2O3(s) +3CO(g) —-> 2Fe(s) + 3CO2(g)
(c) 4BCl3(g) +3LiAlH4(s) ——> 2B2H6(g) + 3LiCl(s) + 3AlCl3(s)
(d) 2K(s) +F2(g)——> 2K+F–(s)
Consider the reactions:
(a) 6CO2(g) 6H2O(l) ———> C6H12O6(s) + 6O6(g) (b) O3(g) + H2O2(l) H2O(l) + 2O2(g)
Why it is more appropriate to write these reactions as:
(a) 6CO2(g) + 12H2O(l) ————-> C6H12O6(s) + 6H2O(l) + 6O2(g)
(b) O3(g) + H2O2 (l) ———–> H2O(l) + O2(g) + O2(g)
Also suggest a technique to investigate the path of above (a) and (b) redox reactions.
Consider the reactions:

Why does the same reductant, thiosulphate react difforerently with iodine and bromine?
Consider the elements: Cs, Ne, I, F
(a) Identify the element that exhibits -ve oxidation state.
(b) Identify the element that exhibits +ve oxidation state.
(c) Identify the element that exhibits both +ve and -ve oxidation states.
(d) Identify the element which neither exhibits -ve nor +ve oxidation state.