

The values of Ksp of two sparingly soluble salts Ni(OH)2 and AgCN are 2.0 x 10-15 and 6 x 10-17 respectively. Which salt is more soluble? Explain.
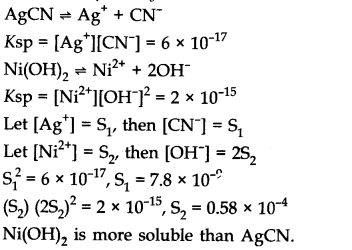
Arrange the following in increasing order of pH.
KN03(aq), CH3COONa(aq), NH4Cl(aq), C6H5COONH4(aq)
Predict which of the following will have appreciable concentration of reactants and products:
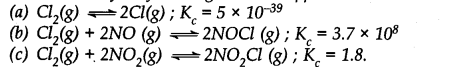
pH of a solution of a strong acid is 5.0. What will be the pH of the solution obtained after diluting the given solution a 100 times?
The degree of ionization of a 0.1 M bromoacetic acid solution is 0.132. Calculate the pH of the solution and the PKa of bromoacetic acid.
The value of Kc for the reaction 2A——>B + C is 2 x 10-3. At a given time, the composition of reaction mixture is [A] = [B] = [C] = 3 x 10-4 M. In which direction the reaction will proceed?
PCl5, PCl3 and Cl2 are at equilibrium at 500 K and having concentration 1.59M PCl5 1.59M Cl2 and 1.41M PCl5. Calculate Kc for the reaction PCl5———>PC13+ Cl2
For the reaction N204(g) ⇌2N02(g), the value of K is 50 at 400 K and 1700 at 500 K. Which of the following options is correct?
(a) The reaction is endothermic.
(b) The reaction is exothermic.
(c) If NO2(g) and N204(g) are mixed 400 K at partial pressures 20 bar and 2 bar respectively, more N204(g) will be formed.
(d) The entropy of the system increases.
A sample of HI (g) is placed in a flask at a pressure of 0.2 atm. At equilibrium partial pressure of HI (g) is 0.04 atm. What is Kp for the given equilibrium?
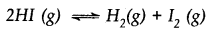
A mixture of 1.57 mol of N2, 1.92 mol of H2 and 8.13 mol of NH3is introduced into a 20 L reaction vessel at 500 K. At this temperature, the equilibrium constant Kc for the reaction
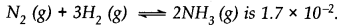
Is this reaction at equilibrium? If not, what is the direction of net reaction?
The value of Kc for the reaction 302(g) —>203(g) is 2.0 x 10-50 at 25 °C. If equilibrium concentration of 02 in air at 25 °C is 1.6 x 10-2, what is the concentration of O3?
Calculate the pH of the following solutions:
(a) 2g ofTlOH dissolved in water to give 2 litre of the solution
(b) 0.3 g of Ca(OH)2 dissolved in water to give 500 mL of the solution
(c) 0.3 g of NaOH dissolved in water to give 200 mL of the solution
(d) l mL of 13.6 M HCl is diluted with water to give 1 litre of the solution.
The solubility product of Al(OH)3 is 2.7 x 10-11. Calculate its solubility in g–L and also find out pH of this solution. (Atomic mass of A1 = 27 u).
The value of Kc for the reaction
2HI(g) ⇌H2(g) + I2(g) is 1 x 10-4. At a given time, the composition of reaction mixture is [HI] = 2 x 10-5 mol, [H2] = 1 x 10-5 mol and [I2] = 1 x 10-5 mol. In which direction will the reaction proceed?
What is the effect of:
(i) addition of H2 (ii) addition of CH3OH
(iii) removal of CO (iv) removal of CH3OH
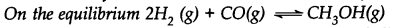
A liquid is in equilibrium with its vapours in a sealed container at a fixed temperature. The volume of the container is suddenly increased, (i) What is the initial effect of the change on the vapour pressure? (ii) How do the rates of evaporation and condensation change initially? (iii) What happens when equilibrium is restored finally and what will be the final vapour pressure?
On the basis of the equation pH = -log [H+], the pH of 10-8 mol dm-3 solution of HC1 should be 8. However, it is observed to be less than 7.0. Explain the reason.
Match the following equilibria with the corresponding condition
| Column I | Column II | ||
| (i) | Liquid⇌Vapour | (a) | Saturated solution |
| (ii) | Solid ⇌Liquid | (b) | Boiling point |
| (iii) | Solid ⇌Vapour | (c) | Sublimation point |
| (iv) | Solute(s) ⇌Solute (solution) | (d) | Melting point ‘ |
| (e) | Unsaturated solution | ||
Hydrogen gas is obtained from the natural gas by partial oxidation with steam as per following endothermic reaction:

Write the expression for Kpfor the above reaction
How will the value of Kp and composition of equilibrium mixture be affected by:
(i) increasing the pressure, (ii) increasing the temperature, (iii) using a catalyst?
Dihydrogen gas is obtained from natural gas by partial oxidation with steam as per following endothermic reaction:
CH4(g) + H2O(g) ——> CO(g) + 3 H2(g)
(a) Write an expression for Kpfor the above reaction.
(b) How will the values of Kp and composition of equilibrium mixture be affected by (i) increasing the pressure (ii) increasing the temperature (iii) using a catalyst?
A reaction between ammonia and boron trifluoride is given below:
:NH3 + BF3 →H3N : BF3
Identify the acid and base in this reaction. Which theory explains it? What is the hybridization of B and N in the reactants?
Which of the following reactions will get affected by increase in pressure ? Also mention whether the change will cause the reaction to go to the right or left direction.
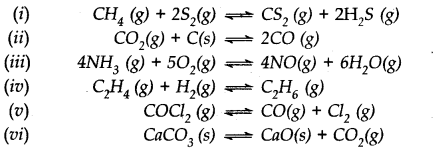
The pH of0.005 M codeine (C18H21N03) solution is 9.95. Calculate the ionization constant and PKb.
(i) Define Le Chatelier’s principle.
(ii) Following reactions occur in a Blast furnace.
Fe203(s) + 3CO(g) ———–>2Fe(s) + 3CO2(g)
use Le chatelier’s principle to predict the direction of reaction when equilibrium mixture is disturbed by
(a) adding Fe203 (b) removing CO2 .
(c) removing CO.
BF3 does not have proton but still acts as an acid and reacts with NH3. Why is it so? What type of bond is formed between the two?
Explain why pure liquids and solids can be ignored while writing the value of equilibrium constants.
What is meant by conjugate acid-base pair? Find the conjugate acid/base for the following species: HNO2, CH–, HClO4 , OH–, CO32-, S2-
The ionization constant of phenol is 1.0 x 10-10. What is the concentration of phenolate ion in 0.05 M solution of phenol? What will be its degree of ionization if the solution is also 0.01 M in sodium phenolate?
Dihydrogen gas used in Haber’s process is produced by reacting methane from natural gas with high temperature steam. The first stage of two stage reaction involves the formation of CO and H2 In second stage, CO formed in first stage is reacted with more steam in water gas shift reaction.

If a reaction vessel at 400 °C is charged with an equimolar mixture of CO and steam so that PCO = PH2O = 4.0 bar, what will be the partial pressure of H2 at equilibrium? Kp = 0.1 at 400 °C.
The values of Ksp of two sparingly soluble salts Ni(OH)2 and AgCN are 2.0 x 10-15 and 6 x 10-17 respectively. Which salt is more soluble? Explain.
The ionization of hydrochloric acid in water is given below:
HCl(aq) + H20(l) ⇌H30 + (aq) +Cl–(aq)
Label two conjugate acid-base pairs in this ionization.
Calculate the volume of water required to dissolve 0.1 g lead (II) chloride to get a saturated solution. (Ksp of PbCl2 = 3.2 x 10-8, atomic mass of Pb = 207 u).
The equilibrium constant for the following reaction is 1.6 x 105at 1024 K.
Find the equilibrium pressure of all gases if 10.0 bar of HBr is introduced into a sealed container at 1024 K.

The pH of a sample of vinegar is 3.76. Calculate the concentration of hydrogen ion in it.
The ionization constant of HF, HCOOH and HCN at 298 K are is 6.8 x 10-4 , 1.8 x 10-4 and 4.8 x 10-9 respectively, Calculate the ionization constant of the corresponding conjugate base.
Assuming complete dissociation, calculate the pH of the following solutions:
(a) 0.003 M HCl (b) 0.005 M NaOH (c) 0.002 M HBr (d) 0.002 M KOH