

A sample of HI (g) is placed in a flask at a pressure of 0.2 atm. At equilibrium partial pressure of HI (g) is 0.04 atm. What is Kp for the given equilibrium?
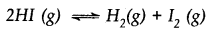

Arrange the following in increasing order of pH.
KN03(aq), CH3COONa(aq), NH4Cl(aq), C6H5COONH4(aq)
pH of a solution of a strong acid is 5.0. What will be the pH of the solution obtained after diluting the given solution a 100 times?
The value of Kc for the reaction 302(g) —>203(g) is 2.0 x 10-50 at 25 °C. If equilibrium concentration of 02 in air at 25 °C is 1.6 x 10-2, what is the concentration of O3?
For the reaction N204(g) ⇌2N02(g), the value of K is 50 at 400 K and 1700 at 500 K. Which of the following options is correct?
(a) The reaction is endothermic.
(b) The reaction is exothermic.
(c) If NO2(g) and N204(g) are mixed 400 K at partial pressures 20 bar and 2 bar respectively, more N204(g) will be formed.
(d) The entropy of the system increases.
Predict which of the following will have appreciable concentration of reactants and products:
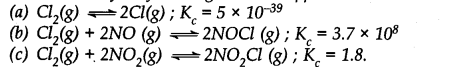
The value of Kc for the reaction 2A——>B + C is 2 x 10-3. At a given time, the composition of reaction mixture is [A] = [B] = [C] = 3 x 10-4 M. In which direction the reaction will proceed?
The degree of ionization of a 0.1 M bromoacetic acid solution is 0.132. Calculate the pH of the solution and the PKa of bromoacetic acid.
The solubility product of Al(OH)3 is 2.7 x 10-11. Calculate its solubility in g–L and also find out pH of this solution. (Atomic mass of A1 = 27 u).
A sample of HI (g) is placed in a flask at a pressure of 0.2 atm. At equilibrium partial pressure of HI (g) is 0.04 atm. What is Kp for the given equilibrium?
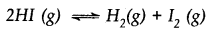
Dihydrogen gas is obtained from natural gas by partial oxidation with steam as per following endothermic reaction:
CH4(g) + H2O(g) ——> CO(g) + 3 H2(g)
(a) Write an expression for Kpfor the above reaction.
(b) How will the values of Kp and composition of equilibrium mixture be affected by (i) increasing the pressure (ii) increasing the temperature (iii) using a catalyst?
On the basis of the equation pH = -log [H+], the pH of 10-8 mol dm-3 solution of HC1 should be 8. However, it is observed to be less than 7.0. Explain the reason.
Hydrogen gas is obtained from the natural gas by partial oxidation with steam as per following endothermic reaction:

Write the expression for Kpfor the above reaction
How will the value of Kp and composition of equilibrium mixture be affected by:
(i) increasing the pressure, (ii) increasing the temperature, (iii) using a catalyst?
A liquid is in equilibrium with its vapours in a sealed container at a fixed temperature. The volume of the container is suddenly increased, (i) What is the initial effect of the change on the vapour pressure? (ii) How do the rates of evaporation and condensation change initially? (iii) What happens when equilibrium is restored finally and what will be the final vapour pressure?
What is the effect of:
(i) addition of H2 (ii) addition of CH3OH
(iii) removal of CO (iv) removal of CH3OH
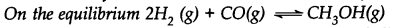
(i) Define Le Chatelier’s principle.
(ii) Following reactions occur in a Blast furnace.
Fe203(s) + 3CO(g) ———–>2Fe(s) + 3CO2(g)
use Le chatelier’s principle to predict the direction of reaction when equilibrium mixture is disturbed by
(a) adding Fe203 (b) removing CO2 .
(c) removing CO.
What is meant by conjugate acid-base pair? Find the conjugate acid/base for the following species: HNO2, CH–, HClO4 , OH–, CO32-, S2-
The ionization constant of HF, HCOOH and HCN at 298 K are is 6.8 x 10-4 , 1.8 x 10-4 and 4.8 x 10-9 respectively, Calculate the ionization constant of the corresponding conjugate base.
The pH of0.005 M codeine (C18H21N03) solution is 9.95. Calculate the ionization constant and PKb.
A reaction between ammonia and boron trifluoride is given below:
:NH3 + BF3 →H3N : BF3
Identify the acid and base in this reaction. Which theory explains it? What is the hybridization of B and N in the reactants?
Explain why pure liquids and solids can be ignored while writing the value of equilibrium constants.
Nitric oxide reacts with bromine and gives nitrosyl bromide as per reaction given below:
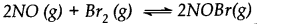
When 0.087 mole of NO and 0.0437 mole of Br2 are mixed in a closed container at constant temperature, 0.0518 mole of NOBr is obtained at equilibrium. Determine the compositions of the equilibrium mixture.
The ionization constant of phenol is 1.0 x 10-10. What is the concentration of phenolate ion in 0.05 M solution of phenol? What will be its degree of ionization if the solution is also 0.01 M in sodium phenolate?
Match the following equilibria with the corresponding condition
| Column I | Column II | ||
| (i) | Liquid⇌Vapour | (a) | Saturated solution |
| (ii) | Solid ⇌Liquid | (b) | Boiling point |
| (iii) | Solid ⇌Vapour | (c) | Sublimation point |
| (iv) | Solute(s) ⇌Solute (solution) | (d) | Melting point ‘ |
| (e) | Unsaturated solution | ||
Assuming complete dissociation, calculate the pH of the following solutions:
(a) 0.003 M HCl (b) 0.005 M NaOH (c) 0.002 M HBr (d) 0.002 M KOH
The values of Ksp of two sparingly soluble salts Ni(OH)2 and AgCN are 2.0 x 10-15 and 6 x 10-17 respectively. Which salt is more soluble? Explain.
BF3 does not have proton but still acts as an acid and reacts with NH3. Why is it so? What type of bond is formed between the two?
A sparingly soluble salt gets precipitated only when the product of concentration of its ions in the solution (Qsp) becomes greater than its solubility product. If the solubility of BaS04 in water is 8 x 10-4 mol dm-3, calculate its solubility in 0.01 mol dm-3 of H2S04.
Which of the following reactions will get affected by increase in pressure ? Also mention whether the change will cause the reaction to go to the right or left direction.
The following concentration were obtained for the formation of NH3 from N2 and H2 at equilibrium at 500 K.[N2(g)] = 1.5 x 10-2 M [H2 (g)] = 3.0 x 10-2 M [NH3] = 1.2 x 10-2 M. Calculate equilibrium constant.
For the equilibrium 2 NOCl(g)——-> 2NO(g) + Cl2(g) the value of the equilibrium constant Kc is 3.75 x 10-6 at 1069 K. Calculate the Kp for the reaction at this temperature?
The equilibrium constant expression for a gas reaction is,
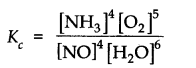
Write the balanced chemical equation corresponding to this expression.
Dihydrogen gas used in Haber’s process is produced by reacting methane from natural gas with high temperature steam. The first stage of two stage reaction involves the formation of CO and H2 In second stage, CO formed in first stage is reacted with more steam in water gas shift reaction.

If a reaction vessel at 400 °C is charged with an equimolar mixture of CO and steam so that PCO = PH2O = 4.0 bar, what will be the partial pressure of H2 at equilibrium? Kp = 0.1 at 400 °C.
Calculate the pH of the following solutions:
(a) 2g ofTlOH dissolved in water to give 2 litre of the solution
(b) 0.3 g of Ca(OH)2 dissolved in water to give 500 mL of the solution
(c) 0.3 g of NaOH dissolved in water to give 200 mL of the solution
(d) l mL of 13.6 M HCl is diluted with water to give 1 litre of the solution.
The value of Kc for the reaction
2HI(g) ⇌H2(g) + I2(g) is 1 x 10-4. At a given time, the composition of reaction mixture is [HI] = 2 x 10-5 mol, [H2] = 1 x 10-5 mol and [I2] = 1 x 10-5 mol. In which direction will the reaction proceed?
A mixture of 1.57 mol of N2, 1.92 mol of H2 and 8.13 mol of NH3is introduced into a 20 L reaction vessel at 500 K. At this temperature, the equilibrium constant Kc for the reaction
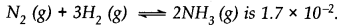
Is this reaction at equilibrium? If not, what is the direction of net reaction?