

(a) What is the limitations of Rutherford model of atoms?
(b) How has Bohr’s theory helped in calculating the energy of hydrogen electron in different energy levels?
(a) Limitations of Rutherford Model:
(i) When a body is moving in an orbit, it achieves acceleration (even if body is moving with constant speed in an orbit, it achieves acceleration due to change in direction). So an electron moving around nucleus in an orbit is under acceleration. However, according to radiation theory of Maxwell, the charged particles when accelerated must emit energy as electromagnetic radiations. This means that the revolving electron must also lose energy continuously in the form of electromagnetic radiation. The loss of energy in revolution of the electron around the nucleus must bring it closer to the nucleus and the electron must ultimately fall into the nucleus by the spiral path. This means that the atom must collapse. But we all know that atom is quite stable in nature.
(ii) Rutherford’s model could not explain the existence of different spectral lines in the hydrogen spectrum.
(b) Based upon the postulates of Bohr’s theory, it is possible to calculate the energy of the hydrogen electron and also one electron species. (He+, Li2+ etc.) The mathematical expression for the energy in the nth orbit is
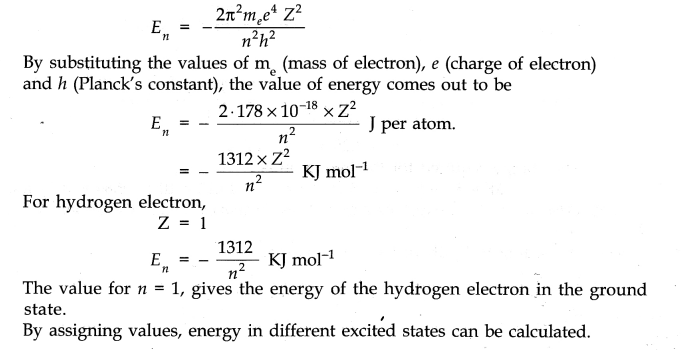
Giue flic name and atomic number of the inert gas atom in which the total number of d-electrons is equal to the difference between the numbers of total p and total s-electrons.
The electronic configuration of valence shell of Cu is 3d10 4s 1 and not 3d94s2. How is this configuration explained?
Which of the following will not show deflection from the path on passing through an electric field?
Proton, cathode rays, electron, neutron.
Give the relation between wavelength and momentum of moving microscopic particle. What is the relation known as?
Define atomic number, mass number and neutron. How are the three related to each other?
Arrange the electron (e), protons (p) and alpha particle (α) in the increasing order for the values of e/m (charge/mass).
Show the distribution of electrons in oxygen atom (atomic number 8) using orbital diagram.
An atom having atomic mass number 13 has 7 neutrons. What is the atomic number of the atom?
What were the weaknesses or limitations of Bohr’s model of atoms ? Briefly describe the quantum mechanical model of atom.
The Balmer series in the hydrogen spectrum corresponds to the transition from n1 = 2 to n2 = 3, 4,……… This series lies in the visible region. Calculate the wave number of line associated with the transition in Balmer series when the electron moves to n = 4 orbit. (RH = 109677 cm-1).
The uncertainty in the position of a moving bullet of mass 10 g is 10-5 m. Calculate the uncertainty in its velocity?
Show the distribution of electrons in oxygen atom (atomic number 8) using orbital diagram.
Arrange s, p and d sub-shells of a shell in the increasing order of effective nuclear charge (Zeff) experienced by the electron present in them
Using Aufbau principle, write the ground state electronic configuration of following atoms.
(i)Boron (Z = 5) (ii) Neon (Z = 10), (iii) Aluminium (Z = 13) (iv) Chlorine (Z = 17), (v) Calcium (Z = 20) (vi) Rubidium (Z = 37)
The uncertainty in the position and velocity of a particle are 10-10m and 5.27 x 10-24 ms-1 respectively. Calculate the mass of the particle. (Haryana Board 2000)
An electron beam after hitting a neutral crystal produces a diffraction pattern? What do you conclude?
State and explain the following:
(i) Aufbau principle
(ii) Pauli exclusion principle.
(iii) Hund’s rule of maximum multiplicity.
The kinetic energy of an electron is 4.55 x 10-25 J. The mass of electron 9.1 x 10-1 kg. Calculate velocity, momentum and the wavelength of the electron?(Haryana Board, 2004, AII CBSE 2000)
What are the two longest wavelength lines (in manometers) in the Lyman series of hydrogen spectrum?
In a hydrogen atom, the energy of an electron in first Bohr’s orbit is 13.12 x 105 J mol-1. What is the energy required for its excitation to Bohr’s second orbit?
An electron beam on hitting a ZnS screen produces a scientillation on it. What do you conclude?
Calculate the de Broglie wavelength of an electron moving with 1% of the speed of light?
(a) What is the limitations of Rutherford model of atoms?
(b) How has Bohr’s theory helped in calculating the energy of hydrogen electron in different energy levels?