

If the radius of the octahedral void is r and radius of the atoms in close-packing is R, derive relation between rand R.
A sphere is fitted into the octahedral void as shown in the diagram.
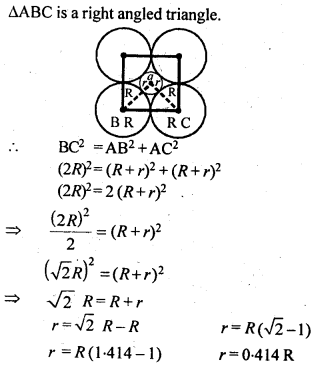
What is the coordination number in a square close packed structure in two dimensions? (a) 2 (b) 3 (c) 4 (d) 6
Under the influence of electric field, which of the following statements are true about the movement of electrons and holes in a p-type semiconductor?
(a) Electron will move towards the positively charged plate through electron holes
(b) Holes will appear to be moving towards the negatively charged plate
(c) Both electrons and holes appear to move towards the positively charged plate
(d) Movement of electrons is not related to the movement of holes
Explain how vacancies are introduced in an ionic solid when a cation of higher valence is added as an impurity in it.
(i) What is meant by the term ‘coordination number’?
(ii) What is the coordination number of atom
(a) in a cubic close-packed structure?
(b) in a body centred cubic structure?
Distinguish between
(i) Hexagonal and monoclinic unit cells
(ii) Face-centred and end-centred unit cells.
Wh ich of the following lattices has the highest packing efficiency (i) simple cubic (ii) body-centered cubic and (iii) hexagonal close-packed lattice?
What type of defect can arise when a solid is heated? Which physical property is affected by it and in what way?
Gold (atomic radius = 0.144 nm) crystallises in a face centred unit cell. What is the length of the side of the unit cell ?
Refractive index of a solid is observed to have the same value along all directions. Comment on the nature of this solid. Would it show cleavage property?
Which of the following statement is not true about the hexagonal close packing?
(a) The coordination number is 12
(b) It has 74% packing efficiency
(c) Tetrahedral voids of the second layer are covered by the spheres of the third layer
(d) In this arrangement, spheres of the fourth layer are exactly aligned with those of the first layer.
Frenkel defect is also known as
(a) stoichiometric defect (b) dislocation defect
(c) impurity defect (d) non-stoichiometric defect
In spite of long range order in the arrangement of particles why are the crystals usually not perfect?
A group 14 element is to be converted into n-type semiconductor by doping it with a suitable impurity. To which group should this impurity belong?
Niobium crystallises in a body centred cubic structure. If density is 8.55 g cm-3, calculate atomic radius of niobium, using its atomic mass 93u.
Explain the following with suitable example:
(i) What is meant by the term coordination number’?
(ii) What is the coordination number of atom
(a) in a cubic close-packed structure?
(b) in a body centred cubic structure?
In which pair most efficient packing is present?
(a) hep and bcc (b) hep and ccp
(c) bcc and ccp (d) bcc and simple cubic cell
What is the two-dimensional coordination number of a molecule in a square close-packed layer?
A compound forms hexagonal close-packed. structure. What is the total number of voids in 0. 5 mol of it? How many of these are tetrahedral voids?
What types of stoichiometric defects are shown by (C.B.S.E. Delhi 2013)
(i) ZnS
(ii) AgBr?
How many lattice points are there is one unit cell of each of the following lattices?
(i) Face centred cubic (if) Face centred tetragonal (iii) Body centred cubic
Ferric oxide crystallises in a hexagonal dose- packed array of oxide ions with two out of every three octahedral holes occupied by ferric ions. Derive the formula of the ferric oxide.
Iodine molecules are held in the crystals lattice by
(a) London forces (b) dipole-dipole interactions
(c) covalent bonds (d) coulombic forces
Which of the following statements are true about semiconductors?
(a) Silicon doped with an electron rich impurity is a p-type semiconductor
(b) Silicon doped with an electron rich impurity is an n-type semiconductor
(c) Delocalised electrons increase the conductivity of doped silicon
(d) An electron vacancy increases the conductivity of type semiconductor
Which of the following defects decrease the density?
(a) Interstitial defect (b) Vacancy defect
(c) Frenkel defect (d) Schottky defect
Explain how many portions of an atom located at
(i)corner and (ii)body centre of a cubic unit cell is part of its neighbouring unit cell.
In terms of band theory, what is the difference
Classify the following as amorphous or crystalline solids: Polyurethane, naphthalene, benzoic acid, Teflon, potassium nitrate, cellophane, polyvinyl chloride, fibreglass, copper
What makes a glass different from a solid such as quartz? Under what conditions could quartz be converted into glass?
How can you determine the atomic mass of an unknown metal if you know its density and the dimensions of its unit cell? Explain.
How many lattice points are there is one unit cell of each of the following lattices?
(i) Face centred cubic (if) Face centred tetragonal (iii) Body centred cubic
Silver crystallises in fcc lattice. If edge length of the cell is 4.07 x 10-8 cm and density is 10.5 g cm-3, calculate the atomic mass of silver.
Which of the following statements are true about metals?
(a) Valence band overlap with conduction band
(b) The gap between valence band and conduction band is negligible
(c) The gap between valence band and conduction band cannot be determined
(d) Valence band may remain partially filled.
The number of tetrahedral voids per unit cell in NaCl crystal is
(c) twice the number of octahedral voids
(d) four times the number of octahedral voids
A perfect crystal of silicon (fig) is doped with some elements as given in the options. Which of these options show n-type semiconductors?


In which of the following arrangements octahedral voids are formed?
(a) hep (b) bcc (c) simple cubic (d) fee
In the following questions, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct answer out of the following choices.
(a) Assertion and Reason both are correct statements and Reason is the correct explanation for Assertion.
(b) Assertion and Reason both are correct statements but Reason is not the correct explanation for Assertion.
(c) Assertion is correct but Reason is wrong.
(d) Assertion is wrong but Reason is correct.
Assertion (A): The total number of atoms present in a simple cubic unit cell is one.
Reason (R): Simple cubic unit cell has atoms at its comers, each of which is shared between eight adjacent unit cells.
Ionic solids, which have anionic vacancies due to metal excess defect, develop colour. Explain with the help of a suitable example.
What makes glass different from a solid such as quartz? Under what conditions could quartz be converted into glass?
If the radius of the octahedral void is r and radius of the atoms in close-packing is R, derive relation between rand R.
What are semi-conductors? Describe the two main types of semiconductors and contrast their conduction mechanisms.
Which of the following lattices has the highest packing efficiency (i) simple cubic (ii) body- centred cubic and (iii) hexagonal close-packed lattice?