

How will you distinguish between the following pairs of terms :
(a) Hexagonal close packing and cubic close packing
(b) Crystal lattice and unit cell
(c) Tetrahedral void and octahedral void.
(a) In hexagonal close packing (hcp), the spheres of the third layer are vertically above the spheres of the first layer
(ABABAB……. type). On the other hand, in cubic close packing (ccp), the spheres of the fourth layer are present above the spheres of the first layer (ABCABC…..type).
(b) Crystal lattice: It deplicts the actual shape as well as size of the constituent particles in the crystal. It is therefore, called space lattice or crystal lattice.
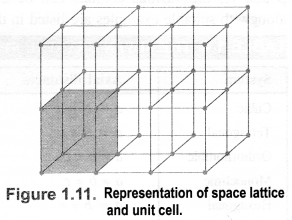 Unit cell: Each bricks represents the unit cell while the block is similar to the space or crystal lattice. Thus, a unit cell is the fundamental building block of the space lattice.
Unit cell: Each bricks represents the unit cell while the block is similar to the space or crystal lattice. Thus, a unit cell is the fundamental building block of the space lattice.
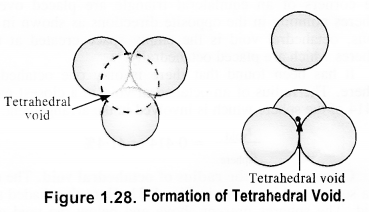
(c) Tetrahedral void: A tetrahedral void is formed when triangular void made by three spheres of a particular layer and touching each other.
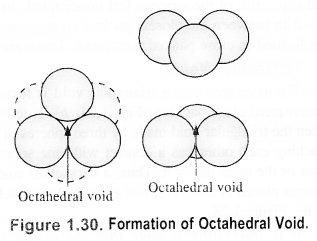
Octahedral void: An octahedral void or site is formed when three spheres arranged at the corners of an equilateral triangle are placed over anothet set of spheres.
Explain how vacancies are introduced in an ionic solid when a cation of higher valence is added as an impurity in it.
‘Stability of a crystal is reflected in the magnitude of its melting points’. Comment. Collect melting points of solid water, ethyl alcohol, diethyl ether and methane from a data book. What can you say about the intermolecular forces between these molecules?
Gold (atomic radius = 0.144 nm) crystallises in a face centred unit cell. What is the length of the side of the unit cell ?
The total number of tetrahedral voids in the face centered unit cell is
(a) 6 (c) 10
(b) 8 (d) 12
A group 14 element is to be converted into n-type semiconductor by doping it with a suitable impurity. To which group should this impurity belong?
Explain how many portions of an atom located at
(i)corner and (ii)body centre of a cubic unit cell is part of its neighbouring unit cell.
A compound forms hexagonal close-packed. structure. What is the total number of voids in 0. 5 mol of it? How many of these are tetrahedral voids?
Explain the following terms with suitable examples :
Ionic solids, which have anionic vacancies due to metal excess defect, develop colour. Explain with the help of a suitable example.
In terms of band theory, what is the difference
(i) What is meant by the term coordination number’?
(ii) What is the coordination number of atom
(a) in a cubic close-packed structure?
(b) in a body centred cubic structure?
Niobium crystallises in a body centred cubic structure. If density is 8.55 g cm-3, calculate atomic radius of niobium, using its atomic mass 93u.
If the radius of the octahedral void is r and radius of the atoms in close-packing is R, derive relation between rand R.
Which of the following is not the characteristic of ionic solids?
(a) Very low value of electrical conductivity in the molten state
(b) Brittle nature
(c) Very strong forces of interactions
(d) Anisotropic nature
In which of the following structure coordination number for cations and anions in the packed structure will be same?
(a) Cl– ions form fee lattice and Na+ ions occupy all octahedral voids of the unit cell.
(b) Ca2+ ions form fee lattice and F- ions occupy all the eight tetrahedral voids of the unit cell
(c) O2- ions form fee lattice and Na+ ions occupy all the eight tetrahedral voids of the unit cell
(d) S2- ions form fee lattice and Zn2+ ions go into alternate tetrahedral voids of the unit cell.
The number of tetrahedral voids per unit cell in NaCl crystal is
(c) twice the number of octahedral voids
(d) four times the number of octahedral voids
Why does the electrical conductivity of semiconductors increase with rise in temperature?
What is the two-dimensional coordination number of a molecule in a square close-packed layer?
What type of defect can arise when a solid is heated? Which physical property is affected by it and in what way?
Explain how vacancies are introduced in an ionic solid when a cation of higher valence is added as an impurity in it.
(i) What is meant by the term ‘coordination number’?
(ii) What is the coordination number of atom
(a) in a cubic close-packed structure?
(b) in a body centred cubic structure?
Silver crystallises in fcc lattice. If edge length of the cell is 4.07 x 10-8 cm and density is 10.5 g cm-3, calculate the atomic mass of silver.
Copper crystallises into a fee lattice with edge length 3.61 x 10-8 cm. Show that the calculated density is in agreement with its measured value of 8.92 gcm-3.
What are semi-conductors? Describe the two main types of semiconductors and contrast their conduction mechanisms.
What makes a glass different from a solid such as quartz? Under what conditions could quartz be converted into glass?
How can you determine the atomic mass of an unknown metal if you know its density and the dimensions of its unit cell? Explain.
A cubic solid is made up of two elements P and Q. Atoms of Q are at the corners of the cube and P at the body centre. What is the formula of the compound? What are the coordination numbers of P and Q?
Copper crystallises into a fee lattice with edge length 3.61 x 10-8 cm. Show that the calculated density is in agreement with its measured value of 8.92 gcm-3.
Under the influence of electric field, which of the following statements are true about the movement of electrons and holes in a p-type semiconductor?
(a) Electron will move towards the positively charged plate through electron holes
(b) Holes will appear to be moving towards the negatively charged plate
(c) Both electrons and holes appear to move towards the positively charged plate
(d) Movement of electrons is not related to the movement of holes
In which of the following arrangements octahedral voids are formed?
(a) hep (b) bcc (c) simple cubic (d) fee
Assertion (A): Semiconductors are solids with conductivities in the intermediate range from

Reason (R): Intermediate, conductivity in semiconductor is due to partially filled valence band.
What makes glass different from a solid such as quartz? Under what conditions could quartz be converted into glass?
Classify each of the following solids as ionic, metallic, modular, network (covalent), or amorphous:
(i) Tetra phosphorus decoxide (P4O10) (ii) Ammonium phosphate, (NH4)3PO4 (iii) SiC (iv) I2 (v) P4 (vii) Graphite (viii), Brass (ix) Rb (x) LiBr (xi) Si
Classify each of the following as being either a p-type or n-type semiconductor :
Which of the following lattices has the highest packing efficiency (i) simple cubic (ii) body- centred cubic and (iii) hexagonal close-packed lattice?
How many lattice points are there is one unit cell of each of the following lattices?
(i) Face centred cubic (if) Face centred tetragonal (iii) Body centred cubic
In the following questions, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct answer out of the following choices.
(a) Assertion and Reason both are correct statements and Reason is the correct explanation for Assertion.
(b) Assertion and Reason both are correct statements but Reason is not the correct explanation for Assertion.
(c) Assertion is correct but Reason is wrong.
(d) Assertion is wrong but Reason is correct.
Assertion (A): The total number of atoms present in a simple cubic unit cell is one.
Reason (R): Simple cubic unit cell has atoms at its comers, each of which is shared between eight adjacent unit cells.
How many lattice points are there is one unit cell of each of the following lattices?
(i) Face centred cubic (if) Face centred tetragonal (iii) Body centred cubic
A cubic solid is made of two elements P and Q. Atoms Q are at the corners of the cube and P at the body centre. What is the formula of the compound ? What is the co-ordination number of P and Q?
Niobium crystallises in a body centred cubic structure. If density is 8.55 g cm-3, calculate atomic radius of niobium, using its atomic mass 93u.
Analysis shows that nickel oxide has the formula Ni0.98 O1.00. What fractions of nickel exist as Ni2+ and Ni3+ ions?