

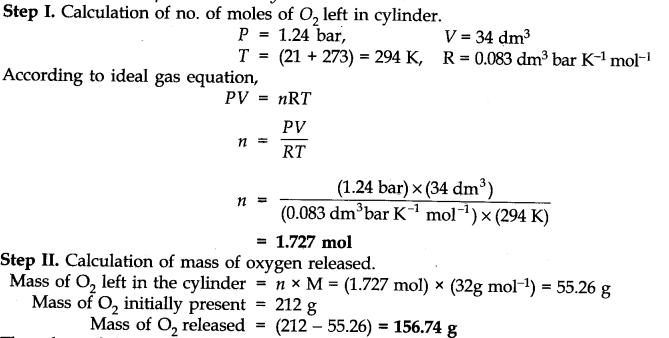
A 34.0 dm3 cylinder contains 212 g of oxygen gas at 21 °C. What mass of oxygen must be released to reduce the pressure in the cylinder to 1.24 bar?
The magnitude of surface tension of liquid depends on the attractive forces between the molecules. Arrange the following in increasing order of surface tension:
water, alcohol (C2H5OH) and hexane [CH3(CH2)4CH3)].
The critical temperature (Tc) and critical pressure (Pc) of C02 are 30.98 °C and 73 atm respectively. Can C02(g) be liquefied at 32 °C and 80 atm pressure?
With regard to the gaseous state of matter which of the following statements are correct?
(a) Complete order of molecules (b) Complete disorder of molecules
(c) Random motion of molecules (d) Fixed position of molecules
Pay load is defined as the difference between the mass of the displaced air and the mass of the balloon. Calculate the pay load when a balloon of radius 10 m, mass 100 kg is filled with helium at 1.66 bar at 27 °C (Density of air = 1.2 kg m-3 and R = 0.083 bar dm3 K-1 mol-1).
Give an expression for the van der Wools equation. Give the significance of the constants used in the equation. What are their units?
For real gases the relation between P, V and T is given by van der Waals equation:

where ‘a' and ‘b' are van der Waals constants, ‘nb' is approximately equal to the total volume of the molecules of a gas.
‘a' is the measure of magnitude of intermolecular attraction.
(i) Arrange the following gases in the increasing order of ‘b'. Give reason. 02, C02, H2, He
(ii) Arrange the following gases in the decreasing order of magnitude of ‘a'. Give reason.CH4, O2, H2
Calculate the temperature of 4.0 moles of a gas occupying 5 dm3 at 3.32 bar (R = 0.083 bar dm3 K-1 mol-1)
Two different gases ˜A' and ˜9' are filled in separate containers of equal capacity under the same conditions of temperature and pressure. On increasing the pressure slightly, the gas ˜A' liquefies but gas ˜B' does not liquefy even on applying high pressure until it is cooled. Explain this phenomenon.
One of the assumptions of kinetic theory of gases is that there is no force of attraction between the molecules of a gas.
State and explain the evidence that shows that the assumption is not applicable for real gases.
Which of the following figures does not represent 1 mole of dioxygen gas at STP?
(a) 16 grams of gas
(b) 22.7 litres of gas
(c) 6.022 x 1023 dioxygen molecules
(d) 11.2 litres of gas
What will be the minimum pressure required to compress 500 dm3 of air at 1 bar to 200 dm3 at 30 °C?
An O2 cylinder has 10 LO2 at 200 atm. If patient takes 0.50 ml of O2 at 1 atm in one breath 37 °C, how many breaths are possible?
One of the assumptions of kinetic theory of gases states that "there is no force of attraction between the molecules of a gas."How far is this statement correct? Is it possible to liquefy an ideal gas? Explain.
What will be the pressure exerted by a mixture of 3.2g of methane and 4.4g of carbon dioxide contained in a 9 dm3 flask at 27 °C?
2.9 g of a gas at 95 °C occupied the same volume as 0.184 g of hydrogen at 17 °C at the same pressure. What is the molar mass of the gas ?
A mixture of dihydrogen and dioxygen at one bar pressure contains 20% by weight of dihydrogen. Calculate the partial pressure of dihydrogen.
Using the equation of state PV = nRT, show that at a given temperature, density of a gas is proportional to the gas pressure P.
Calculate the total pressure in a mixture of 8g of oxygen and 4g of hydrogen confined in a vessel of l dm3 at 27 °C. R = 0.083 bar dm3 K-1 mol-1.
A certain amount of a gas at 27 °C and 1 bar pressure occupies a volume of 25 m3. If the pressure is kept constant and the temperature is raised to 77 °C, what will be the volume of the gas?
Compressibility factor, Z, of a gas is given as Z = PV/nRT
(i) What is the value of Z for an ideal gas?
(ii) For real gas what will be the effect on value of Z above Boyle's temperature?
The pressure of a mixture of H2 and N2 in a container is 1200 torr. The partial pressure of nitrogen in the mixture is 300 torr. What is the ratio of H2 and N2 molecules in the mixture?
State and explain Dalton’s law of partial pressures. Can we apply Dalton's law of partial pressures to a mixture of carbon monoxide and oxygen?
The behaviour of matter in different states is governed by various physical laws. According to you what are the factors that determine the state of matter?
Viscosity of a liquid arises due to strong intermolecular forces existing between the molecules. Stronger the intermolecular forces, greater is the viscosity. Name the intermolecular forces existing in the following liquids and arrange them in the increasing order of their viscosities. Also give reason for the assigned order in one line.Water, Hexane (CH3CH2CH2CH2CH2CH3), Glycerine (CH2OHCH(OH)CH2OH)
What will be the pressure of the gas mixture when 0.5 L of H2 at 0.8 bar and 2.0 L of dioxygen at 0.7 bar are introduced in all vessel at 27 °C?
Density of a gas is found to be 5.46 g/dm3 at 27 °C and at 2 bar pressure. What will be its density at STP?
Assertion (A): Three states of matter are the result of balance between intermolecular forces and thermal energy of the molecules. .
Reason (R): Intermolecular forces tend to keep the molecules together but thermal energy of molecules tends to keep them apart.
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false but R is true.
Assertion (A): Gases do not liquefy above their critical temperature, even on applying high pressure.
Reason (R): Above critical temperature, the molecular speed is high and intermolecular attractions cannot hold the molecules together because they escape because of high speed.
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false but R is true.
Explain the term ‘laminar flow'. Is the velocity of molecules same in all the layers in laminar flow? Explain your answer.
Physical properties of ice, water and steam are very different. What is the chemical composition of water in all the three states?